Intra-abdominal infections generally occurs after entry of enteric organisms into the peritoneal cavity through a defect in the intestinal wall or a viscus as a result of infarction, obstruction, or trauma. Abdominal, retroperitoneal and visceral abscesses generally occur as a complication of local or generalized peritonitis, secondary to appendicitis, diverticulitis, necrotizing enterocolitis, pelvic inflammatory disease, and tubo-ovarian infection, surgery or trauma.
Perforation of the jejunum with a mass
Secondary Peritonitis and Intra abdominal abscesses.
Microbiology
Anaerobic bacteria predominant in intra abdominal infection because they are the main component of the gastrointestinal tract (GIT) flora,1 where they outnumber aerobic and facultative bacteria in the ratio of 1000 to10000 to one.1 The peritonitis that follows introduction of the enteric flora to the peritoneal cavity is usually a synergistic polymicrobial infection.
Number of anaerobic bacteria in the gastrointestinal tract
Number of anaerobic bacteria in the gastrointestinal tract
Secondary Peritonitis: Mixed aerobic and anaerobic flora is recovered in the peritoneal cavity following ruptured appendix, diverticula or intestinal viscus2 and from postoperative wound.3 Similar isolates are also isolated from liver, pelvic and subphrenic abscesses, surgical wounds, and blood cultures of these patients. The predominant aerobic are Escherichia coli, Enterococcus faecalis, Klebsiella spp., Enterobacter spp., and the main anaerobic bacteria are Bacteroides (Bacteroides fragilis group and pigmented Prevotella and Porphyromonas), Peptostreptococcus and Clostridium spp.
Predominant organisms isolate from peritoneal fluid from 100 patients with perforated appendix and 11 patients with postoperative wound infection
| |||
No. of
|
No. of
| ||
isolates
|
isolates
| ||
(No. from
|
(No. from
| ||
Aerobic and facultative
|
wound
|
wound
| |
isolates
|
infection)
|
Anaerobic isolates
|
infection)
|
Gram-positive cocci (total)
|
53 (6)
|
Gram-positive cocci (total)
|
62 (9)
|
Group D enterococcus
|
12 (1)
|
Gram-positive bacilli (total)
|
52 (7)
|
Gram-positive bacilli
|
4 (1)
|
Clostridium sp.
|
16 (2)
|
Gram-negative bacilli (total)
|
87 (10)
|
Gram-negative bacilli
| |
Pseudomonas aeruginosa
|
9 (3)
|
Fusobacterium sp.
|
27 (3)
|
Escherichia coli
|
57 (6)
|
Bacteroides sp.
|
32 (4)
|
Klebsiella pneumoniae
|
7
|
Pigmented Prevotella and Porphyromonas sp.
|
26 (2)
|
B. fragilis group
|
102 (8)
| ||
Total number of aerobes& facultatives
|
144 (17)
|
Total number of anaerobes
|
301 (33)
|
(Modified from Brook, I.: Ann. Surg. 192:208, 1980.)
Liver Abscess: Anaerobes may be involved in at least half of cases of pyogenic liver abscess.4 The most prevalent anaerobes in liver abscess are anaerobic and microaerophilic streptococci, Fusobacterium spp., B. fragilis group, and pigmented Prevotella and Porphyromonas spp. S. aureus and beta-haemolytic streptococci are associated with trauma; Enterococcus spp., K. pneumoniae and Clostridium spp. with biliary disease; and Bacteroides spp. and Clostridium spp. with colonic disease.
Spleenic Abscess: The predominant aerobic and facultative isolates were Escherichia coli , Staphylococcus aureus , Proteus mirabilis , Enterococcus spp., and Klebsiella pneumoniae. 1-5 The predominant anaerobes were Peptostreptococcus spp., Bacteroides fragilis group, Fusobacterium spp. and Clostridium spp. S. aureus, K. pneumoniae and Enterococcus spp., were mostly associated with endocarditis, E. coli with urinary tract and abdominal infection, B. fragilis group and Clostridium spp. with abdominal infection and Fusobacterium spp. with respiratory infection. The most common predisposing causes of splenic abscess are pyogenic infection, splenic trauma, hemoglobinopathies and contiguous disease processes extending to the spleen.
Aerobic and facultative organisms
|
No. of
organisms isolated |
Anaerobic organisms
|
No. of
organisms isolated |
Streptococcus spp. (total)
|
9
|
Peptostreptococcus spp.
|
28
|
Enterococcus spp.
|
4
|
Clostridium spp.
|
14
|
Escherichia coli
|
20
|
Eubacterium spp.
|
2
|
Pseudomonas aeruginosa
|
2
|
Fusobacterium spp.
|
5
|
Klebsiella pneumoniae
|
4
|
Pigmented Prevotella and Porphyromonas spp.
|
7
|
Proteus spp.
|
2
|
Bacteroides spp.
|
7
|
Bacteroides fragilis *
|
14
| ||
Bacteroides thetaiotaomicron *
|
8
| ||
Bacteroides vulgatus *
|
5
| ||
Bacteroides distasonis *
|
2
| ||
Bacteroides ovatus *
|
2
| ||
Total no. of anaerobic and facultative organisms
|
41
|
Total no. of anaerobic organisms
|
91
|
* = Bacteroides fragilis group
Pathogenesis
The dynamics and changes in the microbial flora of the GIT influence the nature and severity of infections after perforation. The number of micro-organisms increases at the distal portions of the GIT. At the stomach and upper bowel there are 104 organisms/g or less, at the lower ileum the number increase to up to 108 organisms/g, and the colon it reaches up to 1011 organisms/g,1 most of which are anaerobes. The changes in the number of intestinal bacteria account for some of the differences in peritoneal cavity cultures after perforations. An average of 3 isolates/specimen and about 107 organisms/g were recovered in perforation of the small intestine, and 26 bacterial isolates and 1012 organisms/g were found in specimens of colonic perforation. The presence of the higher number of organisms in the distal portion of the colon can explain why infection developed in 45% of patients with descending-colon injuries as compared with only 13% in other colon sites.6
The synergistic.relationship between the aerobic and anaerobic bacteria in intraabdominal infections has been demonstrated. 7
Diagnosis
Secondary peritonitis: Fever, diffuse abdominal pain, nausea, and vomiting are characteristic symptoms. Signs of peritoneal inflammation, including rebound tenderness, abdominal wall rigidity, and decrease in bowel sounds are observed. These may be later followed by shock, toxemia, restlessness and irritability, a higher temperature, an increase in the pulse rate, and chills and convulsions.
An elevated blood leukocyte count in excess of 12,000 with a predominance of polymorphonuclear forms is often present. Abdominal roentgenograms may reveal free air in the peritoneal cavity, evidence of ileus or obstruction and obliteration of the shadow of the psoas muscle. CT scanning and ultrasonagrophy can improve the diagnostic accuracy of appendicitis.
Peritonitis
Intra-abdominal abscess: The manifestations of intra-peritoneal abscess that complicate appendicitis are progressive and persistent (> 36 hours) abdominal symptoms, localized peritonitis, systemic toxicity, and a palpable mass on rectal examination, or when right lower quadrant mass is palpated following illness longer than 5 days. Pelvic abscesses can be palpated on rectal examination. Vague upper abdominal pain and pulmonary or pleural symptoms often suggest a subphrenic location.
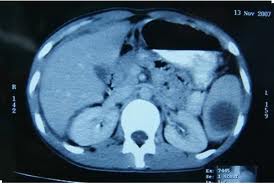
CT of abdominal abscess
Liver abscesses generally presents with fever accompanied by chills, malaise, sweats and aching pain and tenderness over the liver or epigastrium. Leukocytosis, anemia, hypergammaglobulinemia, elevated alkaline phosphatase, and other liver enzymes, and positive blood culture are often present. Splenic abscesses is charachterized by fever, abdominal pain, that is generalized or localized to the left upper quadrant.
CT of liver abscesses
Air fluid level outside the intestinal lumen, localized ileus, or right lower quadrant mass suggest an appendiceal abscess; presence of a soft tissue mass, loss of psoas shadow, or displacement of the ureter or bladder can suggest the presence of retroperitoneal abscess. Subphrenic abscesses are often associated with pleural effusions.
CT of splenic abscess
The best imaging procedure for the diagnosis of abdominal abscesses is CT. 8 Ultrasonically guided fluid collection and abscess drainage are routine procedures in diagnosis and treatment of abdominal abscesses. CT and fluoroscopy with contrast often give invaluable information.
Radiological studies for abscesses may show elevation, change in contour, and reduced mobility of the diaphragm. Left lobe liver Abscess may deform the barium- or gas-filled stomach, or displace the duodenal cap. Pleural effusion or thickening also may be observed, and occasionally a gas-fluid level may be noted inside the liver.
Management
Surgical removal of the inflamed appendix has been the standard of care for appendicitis. A 2015 study illustrated that 3/4 of patients with CT-diagnosed uncomplicated appendicitis treated with antibiotics did not
need to have their appendix surgically removed. Those who eventually needed the
surgery were not harmed by postponing the procedure as there were no
intra-abdominal abscesses or other major complications associated with delayed appendectomy. Because patients with complicated appendicitis, with appendicoliths, children, and pregnant women were excluded from this study, the results do not apply to these groups.
Surgical correction and drainage are important in the treatment of peritoneal and intra-abdominal infection. Surgical intervention should be performed as soon as possible, preferably after the patient is stabilized.
Surgical correction and drainage are important in the treatment of peritoneal and intra-abdominal infection. Surgical intervention should be performed as soon as possible, preferably after the patient is stabilized.
Management of pyogenic intra-peritoneal abscess includes adequate drainage maintenance of fluid, nutritional, and electrolyte status, and systemic antimicrobials.
The effective management is influenced by the location and number of abscesses. In instances of acute appendicitis associated with an abscess, the initial approach usually includes percutaneous drainage of the abscess, medical therapies that includes antibiotic therapy, followed by appendectomy at a later time. Percutaneous aspiration of pyogenic abscesses can aid in the diagnosis and provide guidance in the selection of the proper antibiotic therapy.9
Open surgical drainage is rarely necessary, because of the use of ultrasound and better imaging techniques. However, if the patient’s condition is unstable, or the patient does not respond to antimicrobial therapy, open surgical drainage should be performed.
Solitary large liver abscesses should be drained. However, small abscesses generally resolve after several weeks of antimicrobial therapy, as long as any biliary obstruction has been relieved. Multiple splenic abscesses usually respond to several weeks of antimicrobial therapy alone. However, splenectomy or abscess drainage by laparotomy or percutaneously is the therapy of choice for large abscesses and those that do not resolve with antimicrobial therapy.
The proper management of mixed aerobic and anaerobic infections requires the administration of antimicrobials effective against both aerobic and anaerobic components of the infection.10 If such therapy is not delivered, the infection may progress and serious complications may develop.
Antibiotics effective against B. fragilis group include cefoxitin, metronidazole, the combination of a penicillin plus a beta lactamase inhibitor (e.g. piperacillin,or ticarcillin and tazobactam or clavulanate) and carbapenems (e.g. imipenem, meropenem,) and a newer quinolones (i.e. moxifloxacin). Susceptibility testing of these organism should be done in serious infections.
Therapy directed at the Enterobacteriaceae include aminoglycosides, fourth generation cephalosparin; ( e.g. cefepime, ceftazidine) and quinolones. Triple agent therapy that includes also ampicillin to cover Enterococcus spp. is advocated by some. 10
Single-agent therapy with either cefoxitin, carbapenems or a penicillin plus a beta lactamase inhibitor are effective as combination therapies. The advantage of single-agent therapy is avoiding the ototoxicity and nephrotoxicity of aminoglycosides and may also be less expensive. Single agent may, however, not be always effective against hospital-acquired resistant bacteria. If S. aureus is present in an abscess, anti-staphylococcal agents should be used. If MRSA is present or suspected effective therapy should be given.
In treatment of abdominal abscess antimicrobials, especially when used without surgical drainage, should be given for at least 6-8 weeks. A shorter course, of 4-6 weeks, may be used when good surgical drainage has been achieved.
In 2010 the American Surgical Society and American Society of Infectious Diseases have updated their guidelines for the treatment of abdominal infection.10
The recommendations suggest the following:
For mild-to-moderate community-acquired infections in adults, the agents recommended for empiric regimens are: ticarcillin- clavulanate, cefoxitin, ertapenem, moxifloxacin, or tigecycline as single-agent therapy or combinations of metronidazole with cefazolin, cefuroxime, ceftriaxone, cefotaxime, levofloxacin, or ciprofloxacin. Agents no longer recommended are: cefotetan and clindamycin ( Bacteroides fragilis group resistance) and ampicillin-sulbactam (E. coli resistance) and ainoglycosides (toxicity).
For high risk community-acquired infections in adults, the agents recommended for empiric regimens are: meropenem, imipenem-cilastatin, doripenem, piperacillin-tazobactam, ciprofloxacin or levofloxacin in combination with metronidazole, or ceftazidime or cefepime in combination with metronidazole. Quinolones should not be used unless hospital surveys indicate >90% susceptibility of E. coli to quinolones.
Aztreonam plus metronidazole is an alternative, but addition of an agent effective against gram-positive cocci is recommended. The routine use of an aminoglycoside or another second agent effective against gram-negative facultative and aerobic bacilli is not recommended in the absence of evidence that the infection is caused by resistant organisms that require such therapy.
Empiric use of agents effective against enterococci is recommended and agents effective against methicillin-resistant S. aureus (MRSA ) or yeast is not recommended in the absence of evidence of infection due to such organisms.
Empiric antibiotic therapy for health care-associated intra-abdominal infection should be driven by local microbiologic results. Empiric coverage of likely pathogens may require multidrug regimens that include agents with expanded spectra of activity against gram-negative aerobic and facultative bacilli. These include meropenem, imipenem-cilastatin, doripenem, piperacillin-tazobactam, or ceftazidime or cefepime in combination with metronidazole. Aminoglycosides or colistin may be required.
Antimicrobial regimens for children include an aminoglycoside-based regimen, a carbapenem (imipenem, meropenem, or ertapenem), a beta-lactam/beta-lactamase-inhibitor combination (piperacillin-tazobactam or ticarcillin-clavulanate), or an advanced-generation cephalosporin (cefotaxime, ceftriaxone, ceftazidime, or cefepime) with metronidazole.
Complications
Complications of peritonitis include septic shock, respiratory failure, retroperitoneal or intra-abdominal abscesses, small bowel obstruction from adhesions, fistula, and postsurgical wound infection. Complication of abscesses include multiple organ failure. An abdominal abscess can rarely rupture or hemorrhage into the peritoneal cavity, causing peritonitis.
Pancreatic Abscess
A pancreatic abscess (PA) is a collection of pus resulting from tissue necrosis, liquefaction, and infection. Pancreatic abscess is a late complication of acute necrotizing pancreatitis (ANP), occurring more than 4 weeks after the initial attack. Infected necrosis refers to bacterial contamination of necrotic pancreatic tissue in the absence of abscess formation. 11
Pathophysiology
PA forms through various mechanisms, including fibrous wall formation around fluid collections, penetrating peptic ulcers, and secondary infection of pseudocysts. Patients are at risk for sepsis and death as mortality rate approaches 100% if surgical intervention and drainage are not performed.
Microbiology
The organisms isolated from ANP and PA are aerobic and anaerobic bacteria of enteric origin. The aerobic and facultatives include E. coli, Klebsiella pneumoniae, Enterococcus faecalis, S. aureus, Pseudomonas aeruginosa, Proteus mirabilis, and Streptococcus spp. The anaerobes are Gram-negative anaerobic bacilli, Clostridium spp. and Candida spp. 12 Translocation of enteric bacterial flora accounts for many cases of pancreatic infection.
Diagnosis
Physical findings are nonspecific and include abnormal vital signs consistent with sepsis, abdominal guarding, and rebound tenderness. The presence of prolonged pancreatitis, hemodynamic instability, fever, failure of medical therapy, or the presence of fluid collections suggests the possibility of necrosis and potentially, abscess formation. Collection of pancreatic fluid for via CT-guided needle biopsy can establish the bacterial or fungal etiology. 11
Abdominal contrast-enhanced CT; ultrasound, either endoscopic or transabdominal; andMRI are potential modes for imaging pancreatic necrosis or abscess.
Abdominal contrast-enhanced CT; ultrasound, either endoscopic or transabdominal; and
CT of pancreatic abscess
Management
Surgical drainage followed by placement of indwelling drains is the procedure of choice. CT-guided drainage is an option in those who cannot tolerate an open procedure. Systemic antimicrobials effective against the commonly recovered aerobic and anaerobic should be administrated. ( see previous section) Carbapenems are active against all likely pathogens and penetrate well into pancreatic tissue. Other antibiotics that have been shown to be efficacious in ANP include amikacin, cefuroxime, ceftazidime, and metronidazole. 13
Complications
These include recurrent pancreatitis, bowel obstruction, fistula formation and death.
CHOLANGITIS
Cholangitis is an infection of the biliary tract that can cause significant morbidity and mortality. Choledocholithiasis, biliary tract manipulations and interventions and stents are the most common cause of biliary tract obstruction resulting in cholangitis.
Microbiology
Polymicrobial infections is present 2/3 of patients. The predominant bacteria are Escherichia coli , Enterococcus spp., Klebsiella spp. Enterobacter spp. Clostridium spp. and Bacteroides fragilis group. 14
Diagnosis
Physical examination may reveal fever, icterus, jaundice, and right upper quadrant tenderness. Leukocytosis, hyperbilirubinemia and elevated alkaline phosphatase levels, transaminases and serum amylase are common. Blood culture can be positive in half of the patients. Abdominal ultrasound can be diagnostic. Endoscopic retrograde cholangiopancreatography and percutaneous transhepatic cholangiography may be used for diagnosticlly and therapeutically.
Management
Many patients with acute cholangitis respond to antibiotic therapy; however, patients with severe or toxic cholangitis may not improve and may require emergency biliary drainage. Administration of broad-spectrum intravenous antibiotics ( see previous section) and correction of fluid and electrolyte imbalances constitute essential medical care for cholangitis.
The elevated biliary pressures caused by an obstruction may impair with the biliary secretion of antibiotics. Treatment may require decompression and drainage of the biliary system. Percutaneous transhepatic biliary drainage is another possible nonsurgical method of biliary drainage. For patients with severe cholangitis, endoscopic drainage is the current approach.
REFERENCES
1. Gorbach, S.L.: Intestinal microflora. Gastroenterology 60:1110-29, 1971.
2. Brook, I. : A 12 year study of aerobic and anaerobic bacteria in intra-abdominal and postsurgical abdominal wound infections. Surg Gynecol Obstet.169:387-92.1989.
3. Sanderson, P.J., Wren, M.W.P., Baldwin , A.W.F.: Anaerobic organisms in postoperative wounds. J. Clin. Pathol. 32:143-7, 1979.
5. Westh H, Reines E, Skibsted L. Splenic abscess: a review of 20 cases. Scand J Infect Dis. 22:569-573, 1990.
6. Mandal, A.K., Thadepalli H, Matory E, Lou MA, O'Donnell VA Jr..: Evaluation of antibiotic therapy and surgical techniques in areas of homicidal wounds of the colone. Am. Surg. 50:254-7.1984.
7. Brook, I. , Hunter, V., Walker , R.I.
8. Klatchko, B.A., Schwartz, S.I.: Diagnostic and therapeutic approaches to pyogenic abscess of the liver. Surg Gynecol Obstet 168:332-6, 1989.
9. Gohl J, Gmeinwieser J, Gusinde J. Intraabdominal abscesses. Intervention versus surgical treatment. Zentralbl Chir. 1999;124:187-94.
11. Baron TH, Morgan DE: Acute necrotizing pancreatitis N Engl J Med; 340: 1412-7. 1999.
13. Wyncoll DL: The management of severe acute necrotising pancreatitis: an evidence- based review of the literature. Intensive Care Med ; 25: 146-56. 1999.