Anaerobic bacteria can be recovered in occular infections in adults and children.
CONJUNCTIVITIS
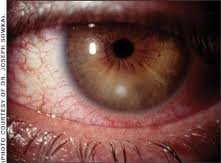
Conjunctivitis is defined as redness of the conjunctivae associated with hyperemia and congestion of the blood vessels, with varying severity of ocular exudate. Preauricular adenopathy may be present. Anaerobic bacteria can be recovered in
Microbiology
The most common aerobic bacteria causing conjunctivitis are Haemophilus influenzae (mostly nontypable), Streptococcus pneumoniae, Streptococcus spp. including Streptoccocus pyogenes, Moraxella species, Staphylococcus aureus, and Staphylococcus epidermidis. Others include Neisseria gonorrhoeae and meningitidis, gram-negative rods such as Pseudomonas and Proteus, and Corynebacterium species.1 The main viral causes are adenovirus, herpes simplex, and Picornavirus.
The most common anaerobe is Peptostreptococcus spp., isolated alone or mixed with other bacteria. 2 Other anaerobes are Bacteroides fragilis, pigmented Prevotella and Porphyromonas, fusobacteria, and bifidobacteria. Anaerobic bacteria were also recovered from patients who wore contact lenses and developed conjunctivitis.3
Pathogenesis
Propionibacterium acnes and Peptostreptococcus spp. are present in the conjunctival sac of uninflamed eyes.4 It is plausible that they become pathogenic under the right circumstances. Oral flora anaerobes can be introduced to the conjunctivae by wetting contact lens with saliva.
Diagnosis
Conjunctivitis associated with anaerobes is indistinguishable from inflammation caused by other bacteria, although patients wearing contact lenses may be at higher risk of developing infections caused by these organisms. Gram and giemsa stains and aerobic and anaerobic cultures are necessary for correct diagnosis.
Management
Conjunctivitis caused by anaerobes should be treated by antimicrobial agents effective against these organisms. Bacitracin is very active against and Peptostreptococcus spp. but is generally inactive against B. fragilis and Fusobacterium nucleatum.5 Erythromycin shows good activity against pigmented Prevotella and Porphyromonas, microaerophilic and anaerobic streptococci, and Gram-positive non—spore-forming anaerobic bacilli. Erythromycin has relatively good activity against Clostridium spp. but poor and inconsistent activity against Gram-negative anaerobic bacilli. Chloramphenicol has the greatest in vitro activity against anaerobes, but should be used cautiously because it is absorbed from the conjunctivae. Anaerobic Gram-positive cocci are the anaerobes most frequently recovered from inflamed conjunctivae and are susceptible to penicillins, macrolides, and chloramphenicol. Anaerobic bacteria may be relatively resistant to sulfonamide, the older quinolones, polymixin B and aminoglyloside preparations that are commonly applied to inflamed conjunctiva. Since anaerobes may be involved in severe cases of conjunctivitis and especially with the most serious complications of bacterial conjunctivitis, such as a penetrating corneal ulcer or orbital cellulitis, special coverage for these organisms should be considered. In such instances administration of parenteral antimicrobial agents should supplement the frequent topical application of medications.
Microbial keratitis is a serious ocular infection that can cause corneal scarring and opacification.
Microbiology
Infective keratitis can be viral, bacterial, fungal and due to Acanthamoeba. The main viruses are herpes simplex, varicella-zoster, measles, mumps, rubella, adenovirus, coxasckievirus A24 and enterovirus 70. Fungal causes are rare and include Aspergillus, Fusarium solani and Candida albicans. A bacterial causes include S. pneumoniae, S. aureus and Staphylococcus epidermidis. Pseudomonas aeruginosa is common in contact lens wearers; H. influenzae and M. catarrhalis cause ulcerative veratitis and enteric organisms (i.e., Shigella, Serratia marcescens) can be transferred by contaminated hands.6
Anaerobic bacteria can also cause keratitis in adults as well as children. The most common one associated with ocular trauma is Clostridium perfringens Clostridium tetani was also rarely described. Other organisms include Peptostreptococcus spp. P. acnes, Propionibacterium avidum Prevotella spp, Fusobacterium spp. and microaerophilic streptococci. 7
Use of contact lenses was associated with the recovery of Pseudomonas spp., Peptostreptococcus spp., Fusobacterium and P. acnes.
Pathogenesis
Predisposing conditions include trauma (e.g., foreign body, corneal laceration, contact lens), corneal exposure (facial palsy, sedated or moribund state, globe prostosis, congenital abnormalities of the eyelids) immune deficiency (immunedeficiency syndrome, immunosuppressive therapy, topical steroids), and abnormalities of ocular surface (dryness, mucin deficiency, vitamin A deficiency, malnutrition, corneal anesthesia).
Diagnosis
The patient presents with severe pain, reflex tearing, eye redness, decreased vision and photophobia. Garyish corneal opacification is characteristic, the light reflex is dulled, and the cornea can be stained with fluorescein. An hypopyon can be observed in the anterior chamber. Corneal scraping of the leading edge and base of ulcer for smears and culture for aerobic and anaerobic and viruses are necessary. Staining with gram and Giemsa is obtained and methenamine-silver, acridine orange and calcoflur white staining are used for detecting fungi and Acanthomoeba.Chlamydia, viruses and some fungi can be detected using recombinant DNA methods, enzyme-linked immunofluorescent assays and fluorescein-labeled monoclonal antibodies.
Management
Topical anti-infective agents are the major therapy. These include a combination of a cephalosporin plus an aminoglycoside, or a quinolone (norfloxacin, ciprofloxacin or ofloxacin).8,9 Frequent administration of topical therapy is important, as they are cleared rapidly. For coverage for anaerobes see the conjunctivitis section.After an initial application of 5 consecutive single drops every minute, and then every 15 minutes for 4 doses, the drops are given every 30 to 60 minutes for at least 2 days. Treatment is continued for 7 to 14 days.
Fungi are treated with frequently administered topical fluocytosine, natamycin, amphotericin B or miconazole for 6 to 12 weeks. Parenteral therapy and excisional keratoplasty is considered when the response is adequate, to prevent deep fungal keratitis and endopthalmitis. Viral infections, excluding herpes, are self-limited and there is currently no effective therapy. Herpes virus infection can be treated with frequently administered (every hour first week, every 2 hours second week) topical antivirals such a vidarabine or trifluorothymidine. Debridement is also an option. Herpes zoster is managed with topical steroids. Acanthamoeba keratitis is treated with the combination of imidazole, propamide isethiocyanate, neomycin and polyhexamethylamine biguanide.
Complications
The corneal transparency may be lost and refractive changes and central corneal scars (leuromas) may occur. Corneal grafting may be necessary.
Dacryocystitis is a bacterial infection of the lacrimal sac. It can occur at any age as a bacterial complication of a viral upper respiratory tract infection (URTI).
Microbiology
S. aureus, S. epidermidis and rarely P. aeruginosa and Escherichia coli have been reported in older patients, while S. pneumoniae, H. influenzae, Streptococcus agalactiae and anaerobes are common in neonates.10 Anaerobic bacteria alone can be recovered in a third of cases, mixed aerobic and anaerobic bacteria in 11% of cases.10 The most frequently recovered anaerobes are Peptostreptococcus, Propionibacterium, Prevotella and Fusobacterium spp. Polymicrobial infection was present in about half of cases.
Pathophysiology
The infection can occur as a result of tear stagnation in the lacrimal sac secondary to obstruction to the normal drainage of the tears through the nasolacrimal duct due to trauma, infection or inflammation, tumor infiltration and after surgery.
Diagnosis
The infection often follow viral URTI, and the patients present with fever, erythema, edema and tenderness over the triangular area below the medial canthus. Purulent material can be expressed from the lacrimal puncta.
Obstruction to drainage can be documented by the dye disappearance test done by instilling 2% sodium fluorescein in the lower conjunctival sac and observing its disappearance after 5 minutes. An alternative method is to irrigate the lacrimal excretory system. However, probing and irrigation should not be done until the inflammation has resolved. Other tests include dacryocystography, CT and MRI.11 Specimen of the pus obtained from the puncta or intraoperatively should be gram-stained and cultured for aerobic and anaerobic bacteria.
Admission to the hospital and parenteral antimicrobial therapy is indicated in acute cases because of the potential for extension of the infection (e.g., cavernous sinus, thrombosis). The choice of therapy depends on the identification of the causative organisms. A first-generation cephalosporin or a beta-lactamase-resistant penicillin (e.g., nafcillin) is adequate for S. aureus. Vancomycin or clindamycin are appropriate in penicillin allergic individuals, and the former for S. aureus resistant to methicillin. Clindamycin, a combination of penicillin plus beta-lactamase inhibitor (e.g., amoxicillin-clavulanate), chloramphenicol, metronidazole (plus a penicillin) or a carbapenem are adequate for anaerobes. When the infection has improved, oral therapy can be substituted for a total of 10 to 14 days.
Incision and draining plus direct application of antibiotics into the sac is indicated to drain a pointed lacrimal sac abscess.11 Surgical drainage is not necessary for most patients; however, probing is helpful in neonates. Probing of the lacrimal excretory system is often sufficient to open the localized membranous obstruction.
Chronic ipsilateral conjunctivitis and corneal ulcers can develop with into the orbit causing orbital abscess. Intraorbital complication should be promptly treated surgically. Delay can lead to visual compromise and life-threatening complications.
ORBITAL AND PERIORBITAL CELLULITIS
Cellulitis of the orbital and periorbital tissues includes a spectrum of disorders that ranges from simple periorbital inflammation to cavernous sinus thrombosis. Orbital cellulitis can be due to hematogenous dissemination, traumatic inoculation, and as a complication of sinusitis.
Bacteremic periorbital cellulitis occurs in children between 6 and 30 months . S. pneumoniae and H. influenzae type b are the most common cause. The introduction of H. influenzae vaccination reduced the rate of this infection..12 In cellulitis related to trauma (including insect bite) or to extension from a neighboring soft tissue area, group A beta-hemolytic streptococci and S. aureus are the most likely pathogens..12Anaerobes could be associated with cellulitis that develops following chronic sinusitis or following sinusitis associated with dental infection. Clostridium perfringens infection can follow a penetrating wound involving a foreign body.
Similar organism are recovered from the infected sinuses and the site of complication.The most common pathogens in cellulitis and orbital abscesses are those seen in acute and chronic sinusitis, depending on the length and etiology of the primary sinusitis. These include S. pneumoniae, H. influenzae, S. aureus, Gram-negative anaerobic bacilli (Prevotella, Porphyromonas, Fusobacterium) ,Peptostreptococcus and microaerophilic streptococci. spp.)13. The organisms isolated in cavernous sinus thrombosis (CST) are S. aureus (50-70% of instances), Streptococcus spp. (20%) and Gram-negative anaerobic bacilli. Similar organisms can be recovered from subperiosteal and orbital abscesses and their corresponding maxillary sinusitis 14.
Pathogenesis
The orbit is separated from the ethmoid cells and maxillary sinus by a thin bony plates ( lamina papyracea). Infections can spread directly by penetration of the thin bones or through the small bony dehiscences. It can also extend directly through the anterior and posterior ethmoid foraminas. Since the ophthalmic venous system has no valves, retrograde thrombophlebitis and spread of the infection can also occur.
Periorbital cellulitis may represent only reactive inflammatory edema in sinusitis. Orbital cellulitis is less common than periorbital cellulitis and involves the globe or orbit. There is diffuse edema of the orbital contents and infiltration of the adipose tissue with inflammatory cells and bacteria. The upper molar or premolar teeth may be the primary site in cases of maxillary sinusitis. Orbital infection may also arise as a metastatic spread of a systemic infection, extension through the orbital septum or through facial veins from a neighboring inflamed soft tissue area, or from a penetrating wound.
Diagnosis
Differential diagnosis include sinus infection, infected periorbital laceration, bacteremic preseptal cellulitis, conjunctivitis, dacryocystitis, systemic or contact allergy, insect bite, seborrheic or eczematoid dermatitis, and nasal vestibular.
Infection in and around the eye must initially be differentiated from trauma, malignancy, dysthyroid exophthalmos, orbital pseudotumor, or cavernous sinus thrombosis.
The severity of the orbital cellulitis is determined by the staging systems of I to V.15 (see Table below)
Distinguishing between infections of the superficial layers and the orbit is critical. The tissue plane separating the two types of infections is a fascial layer termed the orbital septum. Infection anterior to the orbital septum is most described as preseptal cellulitis (periorbital cellulitis). It is characterized by edema, erythema, tenderness, and warmth of the lid (stage I). The eye itself is not involved in preseptal cellulitis and, therefore, the conjunctivae and orbital tissues are not involved. Preauricular lymphadenopathy may be present. Vision, mobility of the globe, and intraocular pressure are normal.
Infection deep to the orbital septum, orbital cellulitis, is characterized by marked lid edema and erythema, proptosis, chemosis, reduction of vision, restriction of mobility of the eye globe in proportion to orbital edema, pain on movement of the globe, fever, and leukocytosis (stage II).54,67 The distinctions between preseptal and orbital cellulitis may be difficult to make. If the infection is allowed to progress, subperiosteal (stage III) or orbital (stage IV) abscess and cavernous sinus thrombosis (stage V) may develop.
Radiographic studies are abnormal if sinusitis is involved. Generally, the ethmoid and maxillary sinuses are involved, but pansinusitis may be present. As clinical examination cannot reliably differentiate between abscess and cellulites, CT is especially useful in defining and localizing the extent of the abscesses and for monitoring of therapy. It should be carried out when an abscess is suspected or when orbital cellulitis has not responded to medical therapy. MRI is reserved for cases where intracranial progression is suspected. Often the swelling of the lid precludes monitoring of the visual acuity and extraocular muscular motility.
When CST involvement is suspected, CT with intravenous contrast material should be done. In cases where improvement is delayed or absent, serial clinical examinations are needed, accompanied by repeated CT, to allow early intervention and drainage. A low threshold needs to be maintained for repeating CT scans after surgical intervention.
Gram stains and cultures for aerobic and anaerobic bacteria should be obtained of any adequately collected purulent material, and blood cultures are imperative. Aspiration and culture of the advancing border of cellulitis may be helpful. In patients with purulent sinusitis, direct aspiration of the sinus can provide bacterial diagnosis.
Preseptal cellulitis
Orbital cellulitis
CT of Right Orbital abscess
CT Right Cavernous sinus thrombosis
Class I
|
Inflammatory oedema and preseptal cellulitis
|
Class II
|
Orbital cellulitis
|
Class III
|
Subperiosteal abscess
|
Class IV
|
Orbital abscess
|
Class V
|
Cavernous sinus thrombosis
|
Medical treatment should be vigorous and aggressive from the early stages of periorbital cellulitis to prevent progression to orbital cellulitis and abscess. If orbital cellulitis or abscess is suspected, an ophthalmologist should be consulted. If rapidly advancing infection is suspected, time is crucial and imaging studies and therapeutic measures should be instituted without delay.
Patients with mild inflammatory eyelid oedema or preseptal cellulitis (class 1) can be treated with oral antibiotics and decongestants. The most effective available oral agents are the second generation cephalosporins or amoxycillin—clavulanate. However, close supervision and follow-up is mandatory, and the initiation of parenteral antimicrobial agents in the hospital should be undertaken if postseptal involvement (classes 2 to 5) is suspected or has developed. The parenteral agents include ceftriaxone or cefotaxime plus coverage for anaerobic bacteria (addition of metronidazole or clindamycin). Drugs that have good brain—blood barrier penetration are preferred.
Anaerobic bacteria should be suspected in periorbital cellulitis associated with dental infections and chronic sinusitis. Antimicrobial agents that generally provide coverage for methicillin-susceptible S. aureus as well as aerobic and anaerobic bacteria include cefoxitin, carbapenems and the combination of a penicillin (e.g. ticarcillin) and a beta-lactamase inhibitor (e.g. clavulanic acid). Metronidazole is administered in combination with an agent effective against aerobic or facultative streptococci and S. aureus. A glycopeptide (e.g. vancomycin) should be administered in cases where methicillin-resistant S. aureus is present or suspected.
Treatment of CST includes high doses of parenteral wide-spectrum antimicrobial agents. The use of anticoagulants and corticosteroids is controversial. Anticoagulants are used to prevent further thrombosis, and the fibrinolytic activity of urokinase helps dissolve the clot. Early diagnosis and vigorous treatment can yield a survival rate of 70–75%. However, permanent sequelae such as blindness and other cranial nerve palsies are common in survivors.
The medical therapy of orbital complications of sinusitis also includes topical and systemic decongestants, humidification, warm compresses, elevation of the head of the bed, analgesics and hydration with intravenous fluids. The patient’s visual acuity and extraocular muscular motility are closely monitored.
Sequential CT
may be needed for follow-up. Cellulitis without an abscess is treated medically. However, if symptoms progress after 24 hours of antibiotics and no improvement occurs after 72 hours, surgical intervention is indicated.
Surgical treatment is mandated by the presence of an abscess on CT, deterioration of visual acuity, signs of deterioration and progression in the orbital involvement despite adequate medical therapy, relapse of symptoms or their progression to the contralateral eye. Surgery involves drainage of the abscess and the involved sinus(es). The indications of a deterioration are radiological or clinical, or both. Drainage should not be delayed, and should be carried out as an emergency treatment. Intranasal endoscopic ethmoidectomy is often utilized to treat subperiosteal abscess. Orbital abscess is still approached with an external incision16. External ethmoidectomy can be reserved to instances in which the orbital signs fail to resolve completely following endoscopic ethmoidectomy, or when visualisation of the ethmoid walls is not possible.
Periorbital and orbital infections pose the risk of serious complications. These include loss of vision owing to involvement of the optic nerve, progression to CST, meningitis, subdural or cerebral abscess, and death.
1. Weiss, A., Brinser, J.H., Nazar-Stewart, V.: Acute conjunctivitis in childhood. J. Pediatr. 122:10–14, 1993.
2. Brook, I., et al.: Anaerobic and aerobic bacteriology of acute conjunctivitis. Ann. Ophthalmol. 11:389, 1979.
3. Brook, I.: Presence of anaerobic bacteria in conjunctivitis associated with wearing contact lens. Ann. Ophthalmol. 20:397–99, 1988.
4. Perkins, R.E., et al.: Bacteriology of normal and infected conjunctivitis. J. Clin. Microbiol. 1:147, 1975.
5. Finegold, S.M.: Anaerobic bacteria in human disease. New York, 1977, Academic Press.
6. Clinch, T.E., Palmon, F.E., Robinson, M.J. et al.: Microbial keratitis in children. Am J Ophthalmol 117:65, 1994.
7. Brook, I., Frazier, E.H.: Aerobic and anaerobic microbiology of keratitis. Ann Ophthalmol 31:21–26, 1999.
8. Groden, L.R., Brinser, J.H.: Outpatient treatment of microbial corneal ulcers. Arch Ophthalmol 104:84, 1986.
9. Parks, D.J., Abrams, D.A., Sarforazi, F.A., Katz, H.: Comparison of topical ciprofloxacin to conventional antibiotic therapy in the treatment of ulcerative keratitis. Am J Ophthalmol 115:471, 1993.
10. Brook, I., Frazier, E.H.: Aerobic and anaerobic microbiology of dacryocystitis. Am J Ophthalmol 125:552–554, 1998.
11. Cahill, K.V., Burns, J.A.: Management of acute dacryocystitis in adults. Ophthalmic Plast Reconstr Surg 9:38–41, 1993.
12. Donahue, S.P., Schwartz, G.: Preseptal and orbital cellulitis in childhood. A changing microbiologic spectrum. Ophthalmology 105:1902–5; discussion 1905–6, 1998.
13. Brook, I., Friedman EM, Rodriguez WJ, Controni G.: Complications of sinusitis in children. Pediatrics 66:568, 1980.
14. Brook, I., Frazier, E.H.: Microbiology of subperiosteal orbital abscess and associated maxillary sinusitis. Laryngoscope. 106:1010–3, 1996.
15. Chandler, J.R., Laagenbrunner, D.J., and Stevens, E.R.: The pathogenesis of orbital complications in acute sinusitis. Laryngoscope 80: 1414, 1970.
16. Manning, S.C.: Endoscopic management of medial subperiosteal orbital abscess. Arch Otolaryngol Head Neck Surg. 119:789–91, 1993.